- Details
- Written by: Germán Fernández
- Category: Biochemistry fundamentals
- Hits: 684
Biochemistry can be defined as the science that deals with the study of the chemical constituents of living cells and the reactions and processes they undergo. Biochemistry also aims to explain the origin of life, a fascinating subject that is still in its early stages.
Life emerged on Earth about three billion years ago in the form of microorganisms with the ability to obtain energy from organic, inorganic, or even sunlight compounds. This energy, along with compounds present on the Earth's surface, allowed for the synthesis of a wide variety of complex organic molecules that, when properly combined, give rise to the complex properties of living organisms.
Chemical Composition of Living Beings
Living beings are composed of different organic and inorganic molecules, with water being the most abundant substance, constituting between 50% and 95% of the cell's weight. Sodium, potassium, magnesium, and calcium cations can represent up to 1%. The remaining weight of living organisms consists of macromolecules composed mainly of carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur, as well as small amounts of other metallic and non-metallic elements.
Most biomolecules have a carbon skeleton to which different groups, called functional groups, are attached, conferring characteristic properties and reactivities. For example, sugars have the carbonyl and hydroxyl functional groups in their structure, while amino acids have the amino and acid groups, and may contain other functional groups in their chain.
Main Types of Biomolecules
Within the cell, we find a large number of small molecules with molecular masses below 10,000 Daltons (D), which can be classified into four families: amino acids, sugars, fatty acids, and nucleotides. One of the functions of these small molecules is to act as monomers in the synthesis of larger molecules (polymers). Amino acids are the basic constituents of proteins; nucleotides make up nucleic acids (DNA and RNA), and small sugar molecules form the long chains of some carbohydrates such as glycogen.
Amino Acids and Proteins
Amino acids are classified as $\alpha$, $\beta$, or $\gamma$ depending on the position of the amino group relative to the carboxylic acid group. $\alpha$-amino acids have the amino group in the position adjacent to the carboxylic acid, from which the side chain also originates. Proteins are formed by 20 $\alpha$-amino acids, although some of them have additional functions, such as glycine and glutamic acid, which also act as neurotransmitters.
However, not all amino acids that are part of living beings are $\alpha$-amino acids. $\gamma$-aminobutyric acid (GABA) is a neurotransmitter found in the brain, and $\beta$-alanine is a precursor of pantothenic acid (vitamin B5).
Amino acids are joined by amide-type bonds, called peptide bonds, to form chains of variable length. Chains with fewer than fifty amino acids are called peptides, while longer polypeptides are called proteins. Proteins perform a wide variety of functions in the cell, including structural, enzymatic, and transport functions, among others.
Sugars and Carbohydrates
Sugars are organic compounds that contain carbonyl and alcohol groups in their structure. Those with an aldehyde function are called aldoses, while those with a ketone function are called ketoses. The most well-known sugar and one of the primary sources of energy for cells is glucose, which consists of six carbon atoms with an aldehyde group at position 1.
These small sugar molecules (glucose, fructose, galactose, ribose, etc.) are called monosaccharides and serve as the monomers for the formation of long-chain sugars or polysaccharides. Polysaccharides are polymers composed of thousands of monosaccharide units.
Glycogen is a polysaccharide formed by glucose units that animals use as an energy storage molecule. Glucose units are extracted from glycogen and "burned" to obtain energy. Other polysaccharides have structural functions, such as cellulose, which makes up the fibers in wood, or chitin, which forms the protective exoskeleton of crustaceans.
Sugars are also found as part of other biomolecules, such as ribose and deoxyribose, which are components of ribonucleic acid (RNA) and deoxyribonucleic acid (DNA). Glycoproteins and glycolipids, abundant on the outer surface of cell membranes, are proteins and lipids bonded to sugar units.
Fatty Acids
Fatty acids are molecules consisting of a carbon chain and a carboxylic acid group at one end. They are monocarboxylic acids (R-COOH). The carbon chain can have double bonds between carbon atoms, in which case they are called unsaturated fatty acids, while those with only single carbon-carbon bonds are called saturated fatty acids.
The carboxylic acid group (-COOH) has a highly acidic hydrogen, and at physiological pH, it is ionized. Therefore, fatty acids are found in cells in the form of carboxylates. For example, oleic acid is found as oleate, and palmitic acid is found as palmitate. It is important to note that the amounts of fatty acids present in cells are minimal; most of them are part of more complex structures such as triglycerides and phosphoglycerides, which are structural components of cell membranes.
Fatty acids are insoluble in water due to their long carbon chain (apolar), although the carboxylic acid group can interact with water molecules, leading to the formation of micelles in the aqueous phase.
Nucleotides and Nucleic Acids
Nucleotides are the units that make up the long chains of nucleic acids. They consist of a sugar (ribose or deoxyribose), a nitrogenous base, and one or more phosphate groups. There are five nitrogenous bases, classified into two families: purines and pyrimidines. Purines are composed of two condensed rings and include adenine (A) and guanine (G). Pyrimidines consist of a single ring and include thymine (T), cytosine (C), and uracil (U).
Nucleotides serve as the structural units of nucleic acids (DNA and RNA), but they also have important energetic functions in the cell. For example, adenosine triphosphate (ATP) stores the energy released during food combustion in the form of phosphate bonds.
Deoxyribonucleic Acid (DNA)
DNA is composed of the sugar deoxyribose and the nitrogenous bases adenine, guanine, cytosine, and thymine. It also contains a phosphate group that connects two nucleotides through a phosphodiester bond. The structure of DNA is a double helix stabilized by hydrogen bonds formed between complementary bases. Adenine from one strand forms hydrogen bonds with thymine from the complementary strand, while guanine pairs with cytosine. A gene is a segment of DNA that contains the information to synthesize a protein. Although DNA is not directly involved in protein synthesis, its instructions are first copied into another molecule called RNA through a process known as transcription.
Ribonucleic Acid (RNA)
RNA is composed of the sugar ribose and the nucleotides adenine, cytosine, guanine, and uracil. Similar to DNA, nucleotides in RNA are connected by phosphodiester bonds, but unlike DNA, which is double-stranded, RNA consists of a single strand. RNA is synthesized by copying a segment of DNA in a process called transcription. The RNA molecule leaves the nucleus and travels to the ribosome, where it translates the information it contains into a sequence of amino acids or a protein.
There are three types of RNA that perform specific roles in protein synthesis: messenger RNA (mRNA), ribosomal RNA (rRNA), and transfer RNA (tRNA). Messenger RNA is formed in the cell nucleus by copying a gene from DNA and carries the necessary information for protein synthesis. Ribosomal RNA, together with proteins, forms ribosomes, supramolecular complexes responsible for linking the amino acids that make up the protein. Finally, transfer RNA is responsible for carrying amino acids to the ribosome. Each transfer RNA molecule specifically binds to an amino acid and possesses a triplet of complementary bases with those of messenger RNA, allowing the pairing of both types of RNA to join the amino acids in the sequence encoded in the mRNA.
- Details
- Written by: Germán Fernández
- Category: Biochemistry fundamentals
- Hits: 622
The enormous diversity of life that exists in nature makes it challenging to answer this question. However, from a biochemical perspective, all living beings behave in a similar manner and obey the same physical and chemical laws. Both a whale and a small microorganism are composed of the same molecules and share numerous biochemical processes.
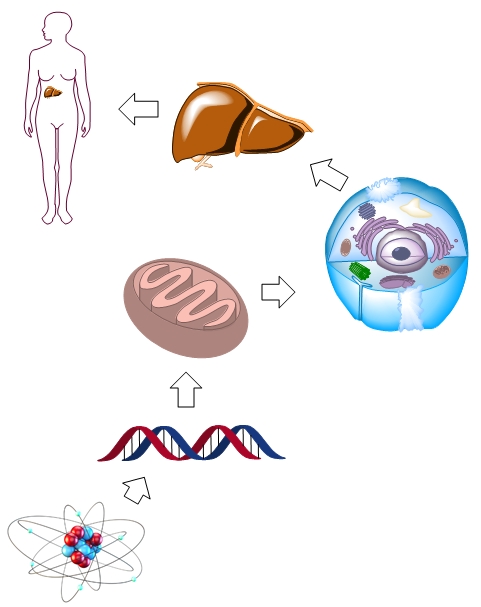 Hierarchical Organization of Multicellular Organisms: Let's examine the main characteristics that define life:
Hierarchical Organization of Multicellular Organisms: Let's examine the main characteristics that define life:
- All living organisms are primarily composed of organic molecules, many of which exhibit high complexity and three-dimensional shapes that determine their function. Life is based on thousands of chemical reactions that occur between these biomolecules and are responsible for vital processes such as growth.
- Living beings are organized into a series of hierarchical levels, and at each level, new properties emerge that did not exist in the previous levels. The most basic level consists of subatomic particles, which form atoms that, in turn, combine to form small biomolecules (amino acids, sugars, nucleotides, etc.). At the next hierarchical level, biomolecules join together to form macromolecules (proteins, nucleic acids, polysaccharides), which can further combine to form even larger structures called supramolecular structures. These supramolecular structures associate to form organelles that make up the cell. In the case of multicellular organisms, there is a higher hierarchy formed by the union of millions of cells, which give rise to organs.
- As we ascend the different levels of organization, new properties emerge that could not be predicted at the previous levels. A visual example of how properties change as we ascend through different levels can be found in iron within hemoglobin. Isolated iron oxidizes in the presence of oxygen; however, the heme group protects it from this oxidation. The properties of iron change when it becomes part of the protein complex. As we ascend the hierarchical levels, new properties emerge that did not exist in the previous levels.
- To maintain a high level of organization and order, living beings are compelled to continuously process matter, expelling waste products in turn. These tasks are carried out through biochemical reactions catalyzed by enzymes. The set of reactions that take place in a living being is called metabolism.
- The cell is composed of organelles enclosed by a cell membrane, which regulates the flow of matter between the interior and exterior of the cell, as well as the cell's response to the extracellular environment. When a cell is divided into its constituents, vital processes cease. Therefore, we can say that the cell is the lowest hierarchical level that exhibits life. On Earth, a cell can only arise from the division of another cell; there is no process that can create a cell from its elemental constituents.
- Life is sustained by the information contained in DNA. The nucleotide sequence of this macromolecule contains the necessary information to synthesize all the proteins required by the cell. Each protein is encoded in a DNA fragment called a gene. When a gene is activated, it transcribes its information into an RNA molecule, which, in turn, translates this information into a protein in the ribosomes. Proteins have a three-dimensional shape that allows them to interact specifically with other molecules that have a complementary spatial shape, and through this interaction, information is transferred.
- An example of information transfer can be found in the interaction between the insulin molecule and receptors present on certain cells' cell membranes. This binding is the signal that indicates to the cell the start of glucose uptake from the extracellular environment.
- Life is in a constant state of evolution and adaptation to the environment. All life on Earth comes from a common ancestor. Diversity and evolution originate from errors that occur during the copying of DNA molecules, known as mutations. Most of these errors are silent, meaning they are either repaired by the cell or have no effect on its function. Other mutations are harmful and reduce the chances of reproduction, causing individuals carrying them to eventually disappear. However, there are mutations that allow organisms to better adapt to the environment, and these individuals reproduce more effectively, leading to population growth. The accumulation of mutations over many generations can give rise to very different forms of life. These life forms continue to adapt to environmental conditions and improve their capabilities to exploit available energy sources.
- Details
- Written by: Germán Fernández
- Category: Biochemistry fundamentals
- Hits: 196
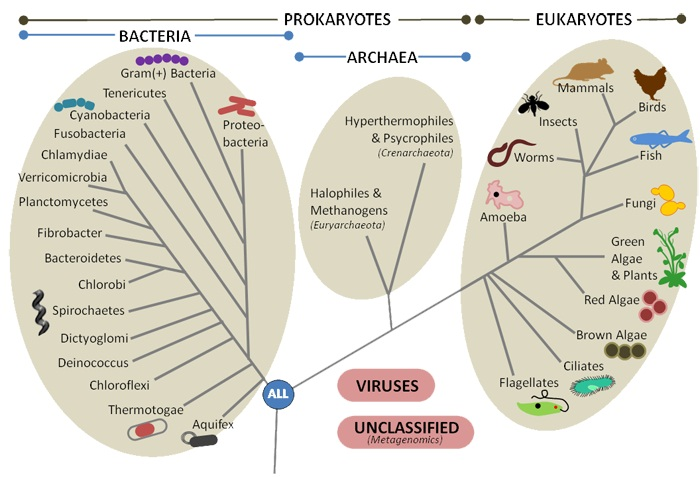
The oldest forms of life on record date back to 3.8 billion years ago and were prokaryotic in nature (cells without a nucleus). Eukaryotic species emerged around 1.8 billion years ago. The current number of living species on Earth is likely in the tens of millions, with the majority being prokaryotic organisms.
Prokaryotic organisms are unicellular and lack a nucleus to separate genetic material from the rest of the cell. It was thought that the prokaryotic world was made up of bacteria, but RNA sequence analysis carried out in the 1980s showed significant genetic differences between two groups of prokaryotes; bacteria and archaea. Although they look similar externally, the biochemical differences are so significant that they necessitate the classification of living beings into three domains: bacteria, archaea, and eukaryotes.
All multicellular living beings (animals, plants, fungi, etc.) are made up of eukaryotic cells, however, the biomass formed by prokaryotes is 10 times greater. Eukaryotic cells are found in soil, water, air, live in the digestive tracts of animals, on the skin, and can even be found several kilometers deep.
- Details
- Written by: Germán Fernández
- Category: Biochemistry fundamentals
- Hits: 210
Metabolism refers to the set of biochemical processes that occur in a living being. It involves thousands of biochemical reactions, each catalyzed by a specific enzyme whose mission is the acquisition of energy, the synthesis of the biomolecules necessary for cell function, growth, and the elimination of waste products.
Although numerous, the chemical reactions that occur in cells have the same mechanisms as those studied in organic chemistry, and they can be classified into a few types of reactions: nucleophilic substitution, elimination, addition, isomerization, and oxidation-reduction.
Nucleophilic substitution reactions
These are organic reactions in which an atom or group of atoms is replaced by another. In this reaction, a substrate participates, which has a leaving group on a carbon with strong positive polarity. This carbon will be attacked by the nucleophile, a species with free pairs and very often with a negative charge. As a result, the leaving group is replaced by the nucleophile.

An example of this type of reaction is the formation of glucose-6-phosphate by nucleophilic attack of the -OH at position 6 of glucose on the phosphorus of ATP, with adenosine diphosphate being the leaving group.
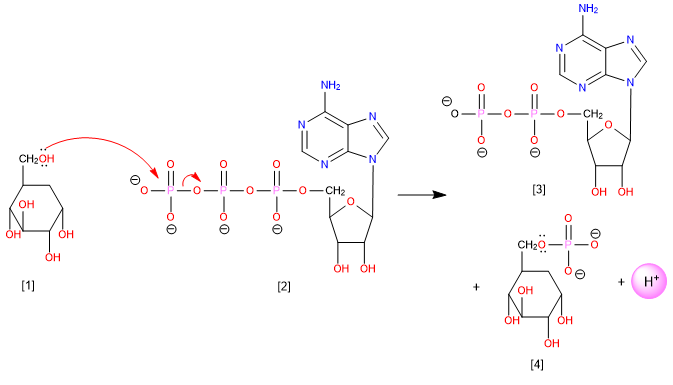
[1] Glucose
[2] Adenosine triphosphate (ATP)
[3] Adenosine diphosphate (ADP)
[4] Glucose-6-phosphate
- Details
- Written by: Germán Fernández
- Category: Biochemistry fundamentals
- Hits: 203
 The cell is a thermal machine capable of generating energy at ambient temperature and pressure, a fundamental fact given the fragility of biological structures. Cells produce most of their energy through redox processes, in which the molecules acting as food are oxidized, losing electrons, which are gained by other molecules with an electron deficiency.
The cell is a thermal machine capable of generating energy at ambient temperature and pressure, a fundamental fact given the fragility of biological structures. Cells produce most of their energy through redox processes, in which the molecules acting as food are oxidized, losing electrons, which are gained by other molecules with an electron deficiency.
This gain or loss of electrons is generally carried out through the exchange of hydrogen radicals or hydride ions. The redox coenzymes FAD (flavin adenine dinucleotide) and NAD+ (nicotinamide adenine dinucleotide) are responsible for removing hydrogens from substrates, transforming into FADH2 and NADH.
- Details
- Written by: Germán Fernández
- Category: Biochemistry fundamentals
- Hits: 193
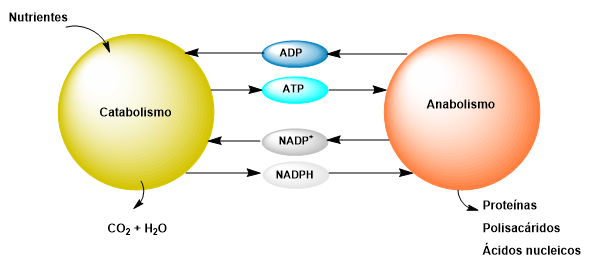
Metabolism encompasses all the biochemical reactions that take place within a cell. These reactions are catalyzed by enzymes, and many of them are coupled, meaning the product of one reaction is the reactant for the next. Therefore, many reactions can be grouped into biochemical pathways.
Pathways dedicated to the biosynthesis of macromolecules from their basic constituents are called anabolic. For example, protein synthesis from amino acids or the synthesis of DNA or RNA from nucleotides. These pathways require an energy input that can come from solar radiation or the degradation of other macromolecules.

