Metabolism refers to the set of biochemical processes that occur in a living being. It involves thousands of biochemical reactions, each catalyzed by a specific enzyme whose mission is the acquisition of energy, the synthesis of the biomolecules necessary for cell function, growth, and the elimination of waste products.
Although numerous, the chemical reactions that occur in cells have the same mechanisms as those studied in organic chemistry, and they can be classified into a few types of reactions: nucleophilic substitution, elimination, addition, isomerization, and oxidation-reduction.
Nucleophilic substitution reactions
These are organic reactions in which an atom or group of atoms is replaced by another. In this reaction, a substrate participates, which has a leaving group on a carbon with strong positive polarity. This carbon will be attacked by the nucleophile, a species with free pairs and very often with a negative charge. As a result, the leaving group is replaced by the nucleophile.

An example of this type of reaction is the formation of glucose-6-phosphate by nucleophilic attack of the -OH at position 6 of glucose on the phosphorus of ATP, with adenosine diphosphate being the leaving group.
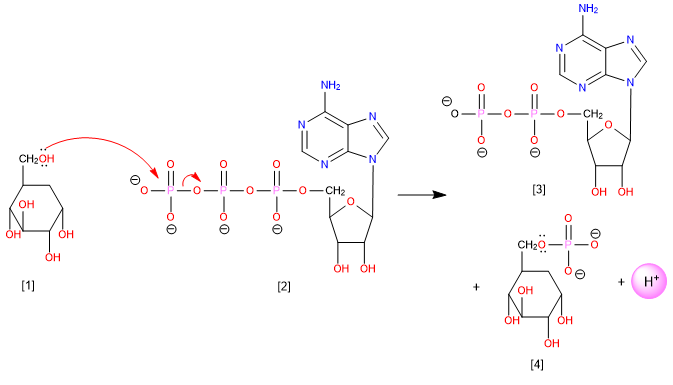
[1] Glucose
[2] Adenosine triphosphate (ATP)
[3] Adenosine diphosphate (ADP)
[4] Glucose-6-phosphate
Another example of a substitution reaction is the hydrolysis of ATP, in which a significant amount of energy is released and serves to drive a wide variety of reactions that require an energy input.
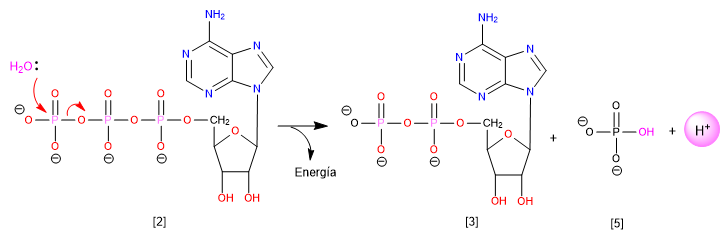
[2] ATP
[3] ADP
[4] Inorganic phosphate
Elimination reactions
These are reactions in which atoms are removed from a molecule and a double bond is formed. The elimination of hydroxyl groups in the form of water molecules is very common, although ammonia, amines, or alcohols can also be eliminated.
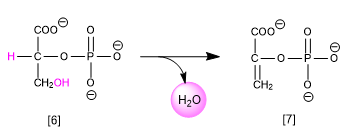
In the metabolism of carbohydrates, we find the elimination of water (dehydration) from 2-phosphoglycerate [6] transforming it into phosphoenolpyruvate [7].
Addition reactions
In addition reactions, two molecules combine to generate a new product. One of the most common biochemical reactions is the addition of water or hydration of an alkene to form an alcohol. In the following example, fumarate is hydrated to malate.
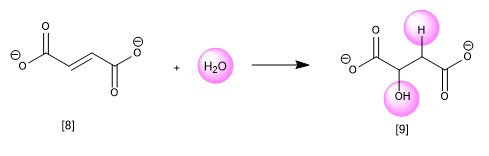
[8] Fumarate
[9] Malate
Isomerization reactions
These are reactions in which rearrangements within a molecule occur, but without gain or loss of atoms. An example is the isomerization between aldoses and ketoses.
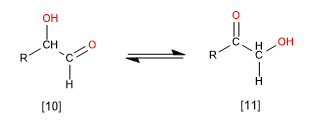
[10] Aldose
[11] Ketose
Oxidation-reduction reactions
These are reactions in which two substances exchange electrons. The substance that donates electrons is oxidized, thus acting as a reducing agent. The substance that accepts electrons is reduced, thus acting as an oxidizing agent.
A simple way to determine if a molecule is oxidized is to see if it gains oxygens or loses hydrogens. If it loses oxygens or gains hydrogens, the molecule is being reduced.
In the following reaction, ethanol is oxidized to acetic acid. In the oxidation process, hydrogens are lost and oxygens are gained.
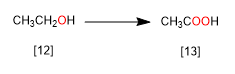
[12] Ethanol
[13] Acetic acid

